How to Bulletproof Your Cannabis SOPs Before Your Next Inspection
Real-world strategies to keep your operation audit-ready,
William Kanistras
4/21/20253 min read


Part of the Cannovise Weekly SOP Series
Every Monday, Cannovise releases a new guide, SOP example, outline, or other usable document designed to help cannabis operators build stronger systems. These free resources are written by real operators, for real operations. Check back weekly to build your complete compliance library over time
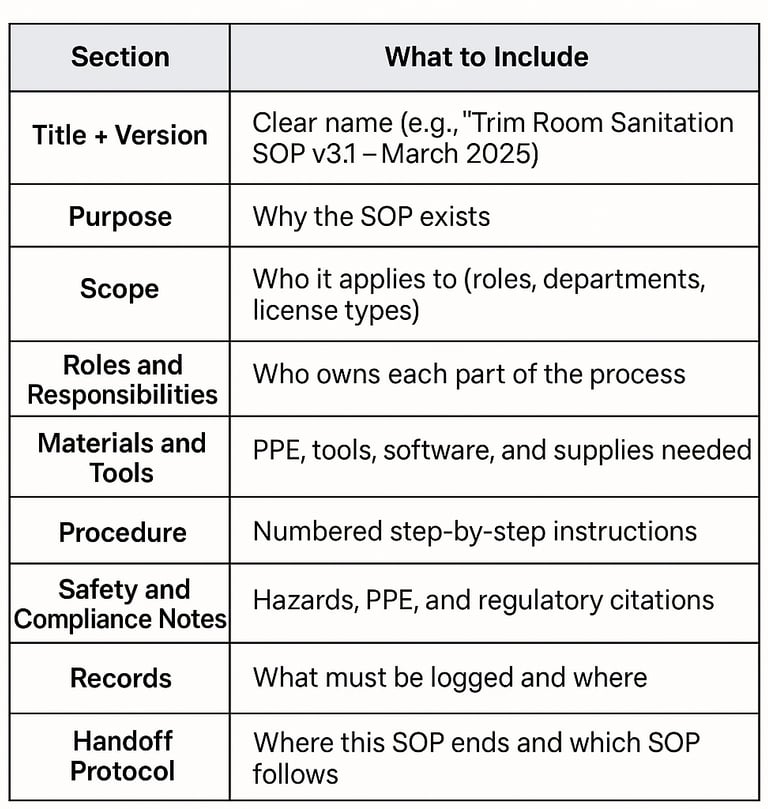
1. Map Before You Write: Start with an Operational Flowchart
Before drafting a single SOP, it is essential to map your process. Begin with an operational flowchart that outlines every key step in your operation, from seed to sale. It helps identify areas where your processes could fail or where regulators are likely to focus during inspections.
Next, overlay your compliance requirements:
Federal: OSHA, EPA (hazmat, workplace safety), ADA
State: Licensing regulations, traceability, packaging
Local: Business permits, fire code, zoning
GMP: Batch control, sanitation, deviation handling
Brand/Investor Requirements: Sustainability protocols, internal quality systems
This is a critical first step in building a comprehensive, compliant, scalable SOP system.
2. Start With the Regs, Not Just a Template
While templates can be useful, it is best not to rely on them alone. Start by clearly citing the actual regulations that apply to your business. Each SOP should reference the relevant legal requirements.
Instead of saying:
“Harvested material should be tagged and logged.”
Use:
“All harvested material must be tagged and logged in Metrc before transport to the drying room. (CCR §15304.1)”
By directly citing regulations, your SOPs demonstrate a clear link to compliance and help ensure the correct steps are followed.
3. Make SOPs Flow, Not Float
A common mistake is treating SOPs as isolated documents. SOPs should be interconnected, especially when one process feeds directly into the next.
Include a handoff protocol at the end of each SOP:
Name and location of the next SOP
Materials or records that need to be transferred
Any required approvals or sign-offs
This creates a seamless workflow across your entire operation.
4. Use Clear “If/Then” Steps, Not Vague Guidelines
SOPs should be written in clear, actionable steps.
Bad:
“Employees should report any issues to management.”
Good:
“If pests are detected in the cultivation area, the IPM Lead must be notified within 1 hour. A Pest Incident Form must be completed and logged in the Cultivation Log by the end of the shift.”
Add decision trees or diagrams for complex processes to enhance clarity.
5. Involve the People Who Do the Work
To make your SOPs realistic and effective, get input from the team members performing the work. Their perspective often reveals what happens on the ground.
Ask them:
What tools do they use?
What problems do they regularly encounter?
What part of the process causes delays or errors?
Incorporate this feedback into your SOPs to improve both compliance and efficiency.
6. Train, Track, and Sign Off
Training is essential to ensure SOPs are followed. Every employee must be trained on the SOPs relevant to their role.
Key steps:
Maintain a training matrix
Collect sign-off sheets
Use retraining protocols when SOPs are updated
These elements will also serve as audit-ready documentation.
7. Maintain Version Control
SOPs must be living documents. As processes evolve, so should your SOPs.
Track:
Version number and date
What changed and why
Who approved the updates
This creates a clear audit trail.
8. Make It Inspection-Friendly
Organize your SOPs for ease of use under pressure.
Best practices:
Include a table of contents
Use consistent naming conventions (e.g., “Cultivation: Harvest SOP”)
Maintain internal versions with staff notes, and separate clean copies for auditors.
9. SOPs and CAPAs: Connecting the Dots
Your SOPs should trigger Corrective and Preventive Actions (CAPAs) when a deviation occurs.
For example:
A pest outbreak triggers a CAPA
The CAPA process is outlined within the SOP
Follow-up actions, root cause analysis, and preventive steps are documented
Integrating CAPA supports both compliance and continuous improvement.
10. SOP Validation and Testing
Validate SOPs by running mock operations.
Ask:
Can staff follow the SOP as written?
Are any regulatory gaps or ambiguities present?
Where are real-world breakdowns likely to happen?
Use feedback to refine your SOPs and improve functionality.
11. Building a Digital SOP Library
Digital SOP systems are efficient and scalable.
Benefits:
Easy updates and version control
Searchable by keyword, department, or function
Cloud-based access for distributed teams
Investing in digital SOP tools can streamline your compliance efforts. (Simplifya is a great cannabis SOP library tool.)
Final Takeaways
SOPs must be more than checklists. They should be functional, real-world tools that drive consistency and compliance.
Map your operation and build from there
Write SOPs that are actionable and interconnected
Train your team and track changes over time
Keep your SOPs updated, validated, and inspection-ready
Need Help With SOPs?
At Cannovise, we build custom SOP libraries that scale with your operation, meet compliance needs, and support real-world workflows. Book a free SOP audit or onboarding consultation to get started.
Stay in the loop
This article is part of the Cannovise Weekly SOP Series. We publish a new guide, template, or SOP resource every Monday. Visit our blog weekly to build your complete compliance library over time.
Your partner in cannabis compliance and operations.
info@cannovise.com
© 2025. All rights reserved.
